How to Find Hydroxide Ion Concentration From Hydronium
The concentration of hydroxide ion in an aqueous solution at 25C is 67 10-4 M. The value of pH will be pH 12565.

Solved B What Is The Hydroxide Ion Concentration Oh In Chegg Com
By signing up youll get.

. Can someone show me how to calculate the hydroxide ion concentration in an aqueous solution that contains 350 x 10-3 M in hydronium ion. Log H -pH and H 10 -pH Step Four Now substitute and evaluateH 10-3. So the pH is the -log of the hydronium ion.
In order for H 3 O OH to remain constant the hydroxide ion concentration must decrease. Calculating Hydronium and Hydroxide Ion Concentration Using Ka and Kb. The hydronium ion concentration may be calculated from the pH by reversing the mathematical method used to get the pH.
POH is defined as the negative logarithm to base 10 of the hydroxide ion concentration in mol L -1 pOH -log 10 OH - OH - is the concentration of hydroxide ions in mol L -1 molL or M. H3O 10-pH or H3O antilog - pH Example. Now substitute and evaluate.
OOH is classified as a negative logarithm to base 10 that indicates -log10OH- when using mol L-1 pOH for a hydroxide ion concentration of -3. To calculate Concentration Of Hydronium ion Using pH you need Negative Log Of Hydronium Concentration pH. To look for the hydroxide ion concentrationwe have to arrange water equilibrium expressionKw 1.
We must rearrange equation pH -log H in order to solve for concentration. On some calculatorsyou obtain the anti logarithm by taking the inverse of the logarithm On othersyou use a 10 x key. See the answer See the answer See the answer done loading.
To find the hydroxide ion concentration in an aqueous solution we have to have the pOH value of the solution. What are the hydronium ion concentration and the hydroxide ion concentration in pure water at 25 C. With our tool you need to enter the respective.
What is the hydronium ion concentration in a solution that has a pH of 834. The Concentration of Hydronium ion using pH formula is defined as 10 to the power negation of pH of the solution is calculated using Hydronium Ion Concentration 10-Negative Log Of Hydronium Concentration. Calculate 10-834 or the inverse log on a calculator 1083.
If a base is added to pure water the reverse is true. This concentration of hydroxide ions can be used to calculate the pOH of solution. 9 x 10-4 MStep 5.
The pH - log H So hydrogen ion concentration is H antilog -pH 10-pH. To calculate pOH Using Concentration Of Hydroxide ion you need Hydronium Ion Concentration C. The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH.
To convert from pH to ion concentrationsfirst apply equation 17-1 to calculate H3O. H3O 10-pH or H3O antilog - pH Example. What is the concentration of the hydronium ion.
PH pOH 14. When known the pOH of the solution can be used to calculate the hydroxide ion concentration in asolution of OH- mol L-1 if a hV-concentration is present. We have to arrange equation pH -log H to be able to solve for concentrationStep 3.
Hydronium ion14 H3O also known as H can be obtained from a known concentration of a hydroxide ion and the equation KwHOH-. On a calculator calculate 10-834 or inverse log 834. Then make use of water equilibrium to calculate OH- Step 2.
From this we can calculate the pH using. POHtext text - textlogleft 00368 right 14341. H 10-305 89 x 10-4 M.
The pOH using concentration of Hydroxide ion formula is defined as the concentration of hydroxyl ion in aqueous solution is calculated using Negative Log Of Hydroxyl Concentration 14 log10 Hydronium Ion Concentration. Even if the pH value is. H3O 10-pH or H3O antilog - pH Example.
What is the hydronium ion concentration in a solution that has a pH of 834. As a result H 3 O OH and the solution is said to be acidic. On a calculator calculate 10-834 or inverse log 834.
What is the hydronium ion concentration in a solution that has a pH of 834. The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. On a calculator calculate 10-834 or inverse log - 834.
Pure water is considered to neutral and the hydronium ion concentration is 10 x 10-7 molL which is equal to the hydroxide ion concentration. Calculate the hydroxide ion concentration in an aqueous solution with a pH of 1147 at 25 degrees Celsius. With our tool you need to enter the respective.
Then utilize water equilibrium to calculate OH-Step Two. The concentration of hydroxide ion in an aqueous solution at 25C is 67 10-4 M. For example is the hydronium ion concentration in a solution with a pH of 834 H3O 10-pH or H3O antilog -pH.
This problem has been solved. The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. What is the concentration of the hydronium ion.
Log H -pH and H 10 -pH. The hydroxide ion concentration in an aqueous solution OH - in mol L -1 can be calculated if the pOH of the solution is known.

Solved An Aqueous Solution Has A Hydroxide Ion Concentration Chegg Com

Calculating Poh From Hydrogen Or Hydroxide Ion Concentration Youtube

Calculating Hydroxide Ion Concentration Youtube

Calculating Hydronium Ion Concentration From Ph And Identifying Molecules Based On Ph R Chemhelp

Ph Value Of A Solution Whose Hydronium Ion Concentration Is A 6 2 Xx 10 9 Mol L Is Youtube

The Connection To Hydronium Ion And Hydroxide Ion Concentrations Youtube

Find Out The Oh Ion Concentration In 100 Ml Of 0 015 M Hcl Is Youtube

Calculating Ph From Hydrogen Or Hydroxide Ion Concentration Youtube

Hydrogen Ion And Hydroxide Ion Concentrations Example Youtube

Converting Between Ph And Hydronium Ion Concentration Chemistry Highschool Chemistry Chemistry Classroom Teaching Chemistry Chemistry Education

Hydrogen Ion And Hydroxide Ion Concentrations Example Youtube

Calculate The Hydronium Ion And Hydroxyl Ion Concentrations In 1 0 001 M Hci Ii 0 01 M Naoh At 298 K Assuming That Both Hcl And Naoh Are Completely Ionised

Oneclass What Is The Hydroxide Ion Concentration Oh In An Aqueous Solution Where The Hydronium

Aleks Interconverting Ph And Hydronium Ion Concentration Youtube
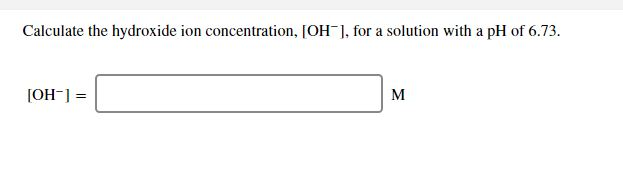
Solved Calculate The Ph Of A Solution That Has A Hydroxide Chegg Com

Pin On Chemistry Worksheets And Task Cards

How To Calculate Hydroxide Ion Oh Concentration From Ph Youtube

Comments
Post a Comment